Lactose intolerance
| Lactose intolerance | |
|---|---|
| Classification and external resources | |
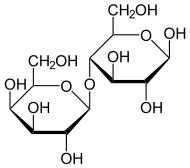 Lactose (disaccharide of β-D-galactose & β-D-glucose) is normally split by lactase. |
|
| ICD-10 | E73. |
| ICD-9 | 271.3 |
| OMIM | 223100 150220 |
| DiseasesDB | 7238 |
| MedlinePlus | 000276 |
| eMedicine | med/3429 ped/1270 |
| MeSH | D007787 |
Lactose intolerance is the inability to metabolize lactose, because of a lack of the required enzyme lactase in the digestive system. It is estimated that 75% of adults worldwide show some decrease in lactase activity during adulthood.[1] The frequency of decreased lactase activity ranges from as little as 5% in northern Europe, up to 71% for Sicily, to more than 90% in some African and Asian countries.[2]
Contents |
Overview
Disaccharides cannot be absorbed through the wall of the small intestine into the bloodstream, so in the absence of lactase, lactose present in ingested dairy products remains uncleaved and passes intact into the colon. The operons of enteric bacteria quickly switch over to lactose metabolism, and the resulting in-vivo fermentation produces copious amounts of gas (a mixture of hydrogen, carbon dioxide, and methane). This, in turn, may cause a range of abdominal symptoms, including stomach cramps, bloating, and flatulence. In addition, as with other unabsorbed sugars (such as sorbitol, mannitol, and xylitol), the presence of lactose and its fermentation products raises the osmotic pressure of the colon contents.
Lactase biology
The normal mammalian condition is for the young of a species to experience reduced lactase production at the end of the weaning period (a species-specific length of time). In humans, in non-dairy consuming societies, lactase production usually drops about 90% during the first four years of life, although the exact drop over time varies widely.[3]
However, certain human populations have a mutation on chromosome 2 which eliminates the shutdown in lactase production, making it possible for members of these populations to continue consumption of fresh milk and other dairy products throughout their lives without difficulty. This appears to be an evolutionarily recent adaptation to dairy consumption, and has occurred independently in both northern Europe and east Africa in populations with a historically pastoral lifestyle.[4] Lactase persistence, allowing lactose digestion to continue into adulthood, is a dominant allele, making lactose intolerance a recessive genetic trait. A noncoding variation in the MCM6 gene has been strongly associated with adult type hypolactasia (lactose intolerance).[5]
Some cultures, such as that of Japan, where dairy consumption has been on the increase compared with traditional dietary patterns, demonstrate a lower prevalence of lactose intolerance in spite of a genetic predisposition.[6]
Pathological lactose intolerance can be caused by coeliac disease, which damages the villi in the small intestine that produce lactase. This lactose intolerance is temporary. Lactose intolerance associated with coeliac disease ceases after the patient has been on a gluten-free diet long enough for the villi to recover (BMJ Textbook of Gastroenterology, Chapter 11, Celiac Disease, Dr.Jamie Gregor & Dr. Diamond Sherin Alidina).
Certain people who report problems with consuming lactose are not actually lactose intolerant. In a study of 323 Sicilian adults, Carroccio et al. (1998) found only 4% were both lactose intolerant and lactose maldigesters, while 32.2% were lactose maldigesters but did not test as lactose intolerant. However, Burgio et al. (1984) found that 72% of 100 Sicilians were lactose intolerant in their study and 106 of 208 northern Italians (i.e., 51%) were lactose intolerant.
Lactose intolerance by group

'</style>
| Human group | Individuals examined | Intolerance (%) | Reference | Allele frequency |
|---|---|---|---|---|
| Basques | 85 | 0.3 | [7] | N/A |
| Dutch | N/A | 1 | [8] | N/A |
| Swedes | N/A | 2 | [9] | 0.14 |
| Europeans in Australia | 160 | 4 | [9] | 0.20 |
| Northern Europeans and Scandinavians | N/A | 5 | [5][10] | N/A |
| British | N/A | 5–15 | [12] | 0.184-0.302[13] |
| Swiss | N/A | 10 | [9] | 0.316 |
| European Americans | 245 | 12 | [9] | 0.346 |
| Tuareg | N/A | 13 | [12] | N/A |
| Germans | N/A | 15 | [12] | N/A |
| Eastern Slavs (Russians, Belarusians, Ukrainians) | N/A | 15 | [14] | N/A |
| Austrians | N/A | 15–20 | [12] | N/A |
| Spaniards (non-Basque) | N/A | 15 | [15] | N/A |
| Northern French | N/A | 17 | [12] | N/A |
| Finns | 134 | 18 | [9] | 0.424 |
| Central Italians | 65 | 19 | [16] | N/A |
| Indians | N/A | 20 | [5][10] | N/A |
| African Tutsi | N/A | 20 | [9] | 0.447 |
| African Fulani | N/A | 23 | [9] | 0.48 |
| Bedouins | N/A | 25 | [12] | N/A |
| Portuguese adults | 102 | 35 | [17] | N/A |
| Southern Italians | 51 | 41 | [16] | N/A |
| African American Children | N/A | 45 | [5] | N/A |
| Saami (in Russia and Finland) | N/A | 25–60 | [18] | N/A |
| Northern Italians | 89 | 52 | [16] | N/A |
| North American Hispanics | N/A | 53 | [12] | N/A |
| Balkans | N/A | 55 | [12] | N/A |
| Mexican American Males | N/A | 55 | [5][10] | N/A |
| Cretans | N/A | 56 | [5] | N/A |
| African Maasai | 21 | 62 | [19] | N/A |
| Southern French | N/A | 65 | [12] | N/A |
| Greek Cypriots | N/A | 66 | [5][10] | N/A |
| Jews, Mizrahi (Iraq, Iran, etc) | N/A | 85 | [20] | N/A |
| Jews, North American | N/A | 68.8 | [5][10] | N/A |
| Jews, Sephardic | N/A | 62 | [20] | N/A |
| Jews, Yemenite | N/A | 44 | [20] | N/A |
| Sicilians | 100 | 71 | [21][22] | N/A |
| South Americans | N/A | 65–75 | [12] | N/A |
| Rural Mexicans | N/A | 73.8 | [5][10] | N/A |
| African Americans | 20 | 75 | [9] | 0.87 |
| Kazakhs from northwest Xinjiang | 195 | 76.4% [23] | ||
| Lebanese | 75 | 78 | [24] | N/A |
| Central Asians | N/A | 80 | [12] | N/A |
| Alaskan Eskimo | N/A | 80 | [5][10] | N/A |
| Australian Aborigines | 44 | 85 | [9] | 0.922 |
| Inner Mongolians | 198 | 87.9 | [23] | |
| African Bantu | 59 | 89 | [9] | 0.943 |
| Asian Americans | N/A | 90 | [5][10] | N/A |
| Northeastern Han Chinese | 248 | 92.3 | [23] | |
| Chinese | 71 | 95 | [9] | 0.964 |
| Southeast Asians | N/A | 98 | [5][10] | N/A |
| Thais | 134 | 98 | [9] | 0.99 |
| Native Americans | 24 | 100 | [9] | 1.00 |
The statistical significance varies greatly depending on number of people sampled.
Lactose intolerance levels also increase with age. At ages 2 – 3 yrs., 6 yrs., and 9 - 10 yrs., the amount of lactose intolerance is, respectively:
- 6% to 15% in white Americans and northern Europeans
- 18%, 30%, and 47% in Mexican Americans
- 25%, 45%, and 60% in black South Africans
- approximately 10%, 20%, and 25% in Chinese and Japanese
- 30–55%, 90%, and >90% in Mestizos of Peru[25][26]
Chinese and Japanese populations typically lose between 20 and 30 percent of their ability to digest lactose within three to four years of weaning. Some studies have found that most Japanese can consume 200 ml (8 fl oz) of milk without severe symptoms (Swagerty et al., 2002).[6]
Ashkenazi Jews can keep 20 - 30 percent of their ability to digest lactose for many years.[8][25][27] Of the 10% of the Northern European population that develops lactose intolerance, the development of lactose intolerance is a gradual process spread out over as many as 20 years.[28]
Diagnosis
To assess lactose intolerance, the intestinal function is challenged by ingesting more dairy than can be readily digested. Clinical symptoms typically appear within 30 minutes but may take up to 2 hours, depending on other foods and activities.[29] Substantial variability of the clinical response (symptoms of nausea, cramping, bloating, diarrhea, and flatulence) is to be expected, as the extent and severity of lactose intolerance varies between individuals.
When considering the need for confirmation, it is important to distinguish lactose intolerance from milk allergy, which is an abnormal immune response (usually) to milk proteins. Since lactose intolerance is the normal state for most adults on a worldwide scale and is not considered a disease condition, a medical diagnosis is not normally required. However, if confirmation is necessary, three tests are available.
Hydrogen breath test
In a hydrogen breath test, after an overnight fast, 50 grams of lactose (in a solution with water) is swallowed. If the lactose cannot be digested, enteric bacteria metabolize it and produce hydrogen. This, along with methane, can be detected in the patient's breath by a clinical gas chromatograph or a compact solid state detector. The test takes about 2 to 3 hours. A medical condition with similar symptoms is fructose malabsorption.
In conjunction, measuring the blood glucose level every 10 – 15 minutes after ingestion will show a "flat curve" in individuals with lactose malabsorption, while the lactase persistent will have a significant "top", with an elevation of typically 50 to 100% within 1 – 2 hours. However, given the need for frequent blood draws, this approach has been largely supplanted by breath testing.
Stool acidity test
This test can be used to diagnose lactose intolerance in infants, for whom other forms of testing are risky or impractical.[30]
Intestinal biopsy
An intestinal biopsy can confirm lactose intolerance following discovery of elevated hydrogen in the hydrogen breath test.[31] However, given the invasive nature of this test, and the need for a highly specialized laboratory to measure lactase enzymes or mRNA in the biopsy tissue, this approach is used almost exclusively in clinical research.
History of diagnosis
The ancient Greek physician Hippocrates (460-370 B.C.) first noted gastrointestinal upset and skin problems in some who consumed milk;[32] patients experiencing the former symptom may likely have been suffering from lactose intolerance. However, it was only in the 20th century that the syndrome was more widely described by modern medical science.
The condition was first recognized in the 1950s and 1960s when various organizations like the United Nations began to engage in systematic famine-relief efforts in countries outside Europe for the first time. Holzel et al. (1959) and Durand (1959) produced two of the earliest studies of lactose intolerance. As anecdotes of embarrassing dairy-induced discomfort increased, the First World donor countries could no longer ascribe the reports to spoilage in transit or inappropriate food preparation by the Third World recipients.
Because the first nations to industrialize and develop modern scientific medicine were dominated by people of European descent, adult dairy consumption was long taken for granted. Westerners for some time did not recognize that the majority of the human ethno-genetic groups could not consume dairy products during adulthood. The term Milk drinking syndrome stands for a phenomenon when European experience is extrapolated to the other populations of the world.[33]
Since then, the relationship between lactase and lactose has been thoroughly investigated in food science due to the growing market for dairy products among non-Europeans.
Originally it was hypothesised that gut bacteria such as E. coli produced the lactase enzyme needed to cleave lactose into its constituent monosaccharides, and thus become metabolisable and digestible by humans. Some form of human-bacteria symbiosis was proposed as a means of producing lactase in the human digestive tract.
Nomenclature
According to Heyman (2006), approximately 70% of the global population cannot tolerate lactose in adulthood. Thus, some argue that the terminology should be reversed — lactose intolerance should be seen as the norm, and the minority groups should be labeled as having lactase persistence. A counter-argument to this is that the cultures that don't generally consume unmodified milk products have little need to discuss their intolerance to it, leaving the cultures for which lactose intolerance is a significant dietary issue to define its terminology.
History of genetic prevalence
The ability to digest lactose into adulthood (lactase persistence) would have only been useful to humans after the invention of animal husbandry and the domestication of animal species that could provide a consistent source of milk. Hunter-gatherer populations before the Neolithic revolution were overwhelmingly lactose intolerant,[34][35] as are modern hunter-gatherers. Genetic studies suggest that the oldest mutations associated with lactase persistence only reached appreciable levels in human populations in the last ten thousand years.[36][37] Therefore lactase persistence is often cited as an example of both recent human evolution[38][39] and, as lactase persistence is a genetic trait but animal husbandry a cultural trait, gene-culture coevolution.[40]
Several genetic markers for lactase persistence have been identified, and these show that the allele has multiple origins in different parts of the world (i.e. it is an example of convergent evolution).[41] The version of the allele most common amongst Europeans is estimated to have risen to significant frequencies about 7,500 years ago in Central Europe, a place and time approximately corresponding to the archaeological Linearbandkeramik culture.[42] Since North Africans also possess this version of the allele it is probable that it actually originated earlier, in the Near East, but that the earliest farmers did not have high levels of lactase persistence and, subsequently, did not consume significant amounts of unprocessed milk.[43] Lactase persistence in Sub-Saharan Africa almost certainly had a separate origin, probably more than one,[44] and it is also likely that there was a separate origin associated with the domestication of the Arabian camel.[45] None of the mutations so far identified have been shown to be causal for the lactase persistence allele, and it is thought that there are several more yet to be discovered.[46]
The evolutionary processes driving the rapid spread of lactase persistence in some populations are not known.[41] In some East African ethnic groups lactase persistence has gone from negligible to near-ubiquitous frequencies in just three thousand years, suggesting a very strong selective pressure.[38][39] But some models for the spread of lactase persistence in Europe attribute it primarily to a form of genetic drift.[47] Competing theories on why the ability to digest lactose might be selected for include nutritional benefits, milk as a water source in times of draught, and increased calcium absorption helping to prevent rickets and osteomalacia in low-light regions.[41]
Roman authors recorded that the people of northern Europe, particularly Britain and Germany, drank unprocessed milk. This corresponds very closely with modern European distributions of lactose intolerance, where the people of Britain, Germany and Scandinavia have a good tolerance, and those of southern Europe, especially Italy, have a poorer tolerance.[48]
In east Asia, historical sources also attest that the Chinese did not consume milk, whereas the nomads that lived on the borders did. Again, this reflects modern distributions of intolerance. China is particularly notable as a place of poor tolerance, whereas in Mongolia and the Asian steppes horse milk is drunk regularly. This tolerance is thought to be advantageous, as the nomads do not settle down long enough to process mature cheese. Given that their prime source of income is generated through horses, to ignore their milk as a source of calories would be greatly detrimental. The nomads also make an alcoholic beverage, called Kumis, from horse milk, although the fermentation process reduces the amount of lactose present.
Managing lactose intolerance
For persons living in societies where the diet contains relatively little dairy, lactose intolerance is not considered a condition that requires treatment. However, those living among societies that are largely lactose-tolerant may find lactose intolerance troublesome. Although there are still no methodologies to reinstate lactase production, some individuals have reported their intolerance to vary over time (depending on health status and pregnancy[49]).
Lactose intolerance is not usually an all-or-nothing condition: the reduction in lactase production—and hence, the amount of lactose that can be tolerated—varies from person to person. Since lactose intolerance poses no further threat to a person's health, managing the condition consists of minimizing the occurrence and severity of symptoms. Berdanier and Hargrove recognise four general principles: avoidance of dietary lactose, substitution to maintain nutrient intake, regulation of calcium intake, and use of enzyme substitute.[31]
Avoiding lactose-containing products
Since each individual's tolerance to lactose varies, according to the US National Institute of Health, "Dietary control of lactose intolerance depends on people learning through trial and error how much lactose they can handle."[50] Label reading is essential, as commercial terminology varies according to language and region.[31]
Lactose is present in two large food categories: conventional dairy products, and as a food additive (in dairy and non dairy products).
Dairy products
Lactose is a water-soluble molecule. Therefore fat percentage and the curdling process have an impact on which foods may be tolerated. After the curdling process, lactose is found in the water portion (along with whey and casein) but is not found in the fat portion. Dairy products which are "fat reduced" or "fat free" generally have a slightly higher lactose percentage. Additionally, low fat dairy foods also often have various dairy derivatives such as milk solids added to them to enhance sweetness, increasing the lactose content.
Milk. Human milk has the highest lactose percentage at around 9%. Unprocessed cow milk has 4.7% lactose. Unprocessed milk from other bovids contains similar lactose percentages (goat milk 4.1%,[51] buffalo 4.86%,[52] yak 4.93%,[53] sheep milk 4.6%)
Butter. The butter-making process separates the majority of milk's water components from the fat components. Lactose, being a water soluble molecule, will still be present in small quantities in the butter unless it is also fermented to produce cultured butter.
Yogurt and kefir. People can be more tolerant of traditionally made yogurt than milk, because it contains lactase enzyme produced by the bacterial cultures used to make the yogurt. However, many commercial brands contain milk solids, increasing the lactose content.
Cheeses. Traditionally made hard cheese (such as Emmental) and soft ripened cheeses may create less reaction than the equivalent amount of milk because of the processes involved. Fermentation and higher fat content contribute to lesser amounts of lactose. Traditionally made Emmental or Cheddar might contain 10% of the lactose found in whole milk. In addition, the traditional aging methods of cheese (over 2 years) reduces their lactose content to practically nothing. [54] Commercial cheese brands, however, are generally manufactured by modern processes that do not have the same lactose reducing properties, and as no regulations mandate what qualifies as an "aged" cheese, this description does not provide any indication of whether the process used significantly reduced lactose.
Sour cream and ice cream, like yogurt, if made the traditional way, may be tolerable, but most modern brands add milk solids.[55] Consult labels.[56]
Examples of lactose levels in foods. As scientific consensus has not been reached concerning lactose percentage analysis methods [57] (non-hydrated form or the mono-hydrated form), and considering that dairy content varies greatly according to labeling practices, geography and manufacturing processes, lactose numbers may not be very reliable. The following are examples of lactose levels in foods which commonly set off symptoms.[50] These quantities are to be treated as guidelines only.
-
Dairy product Lactose content Yogurt, plain, low-fat, 240 mL 5 g Milk, reduced fat, 240 mL 11 g Swiss cheese, 28 g 1 g Ice cream, 120 mL 6 g Cottage cheese, 120 mL 2–3 g
Lactose in non-dairy products
Lactose (also present when labels state lactoserum, whey, milk solids, modified milk ingredients, etc.) is a commercial food additive used for its texture, flavour and adhesive qualities, and is found in foods such as processed meats[58] (sausages/hot dogs, sliced meats, pâtés), gravy stock powder, margarines[59] sliced breads,[60][61] breakfast cereals, potato chips,[62] processed foods, medications, pre-prepared meals, meal replacement (powders and bars), and protein supplements (powders and bars).
Kosher products labeled pareve are free of milk. However, if a "D" (for "Dairy") is present next to the circled "K", "U", or other hechsher, the food likely contains milk solids[58] (although it may also simply indicate that the product was produced on equipment shared with other products containing milk derivatives).
Alternative products
Plant-based milks and derivatives are inherently lactose free: soy milk, rice milk, almond milk, hazelnut milk, oat milk, hemp milk, peanut milk, horchata.
The dairy industry has created low-lactose or lactose-free products to replace regular dairy. Lactose-free milk can be produced by passing milk over lactase enzyme bound to an inert carrier; once the molecule is cleaved, there are no lactose ill-effects. A form is available with reduced amounts of lactose (typically 30% of normal), and alternatively with nearly 0%.
Finland, where approximately 17% of the Finnish-speaking population has hypolactasia,[63] has had "HYLA" (acronym for hydrolysed lactose) products available for many years. These low-lactose level cow's milk products, ranging from ice cream to cheese, use a Valio patented chromatographic separation method to remove lactose. The ultra-pasteurization process, combined with aseptic packaging, ensures a long shelf-life.
Recently, the range of low-lactose products available in Finland has been augmented with milk and other dairy products (such as ice cream, butter, and buttermilk) that contain no lactose at all. The remaining about 20% of lactose in HYLA products is taken care of enzymatically. These typically cost slightly more than equivalent products containing lactose. Valio also markets these products in Sweden and in Estonia.
In the UK, where an estimated 15% of the population are affected by lactose intolerance, Lactofree produces milk, cheese, and yogurt products which contain only 0.03% lactose.
Alternatively, a bacterium such as L. acidophilus may be added, which affects the lactose in milk the same way it affects the lactose in yogurt (see above).
Lucerne, Safeway's dairy brand, produces 100% lactose-free milk. The milk's only noticeable difference from regular milk is a slightly sweeter taste due to the adding of the lactase enzyme. It does not however contain more sugar, and is nutritionally identical to regular milk.
Lactase supplementation
When lactose avoidance is not possible, or on occasions when a person chooses to consume such items, then enzymatic lactase supplements may be used.[64][65]
Lactase enzymes similar to those produced in the small intestines of humans are produced industrially by fungi of the genus Aspergillus. The enzyme, β-galactosidase, is available in tablet form in a variety of doses, in many countries without a prescription. It functions well only in high-acid environments, such as that found in the human gut due to the addition of gastric juices from the stomach. Unfortunately, too much acid can denature it,[66] and it therefore should not be taken on an empty stomach. Also, the enzyme is ineffective if it does not reach the small intestine by the time the problematic food does. Lactose-sensitive individuals can experiment with both timing and dosage to fit their particular needs.
While essentially the same process as normal intestinal lactose digestion, direct treatment of milk employs a different variety of industrially produced lactase. This enzyme, produced by yeast from the genus Kluyveromyces, takes much longer to act, must be thoroughly mixed throughout the product, and is destroyed by even mildly acidic environments. Its main use is in producing the lactose-free or lactose-reduced dairy products sold in supermarkets.
Enzymatic lactase supplementation may have an advantage over avoiding dairy products, in that alternative provision does not need to be made to provide sufficient calcium intake, especially in children.[67]
Rehabituation to dairy products
For healthy individuals with secondary lactose intolerance, it may be possible in some cases for the bacteria in the large intestine to adapt to an altered diet and break down small quantities of lactose more effectively[68] by habitually consuming small amounts of dairy products several times a day over a period of time. Reintroducing dairy in this way to people who have an underlying or chronic illness, however, is not recommended, as certain illnesses damage the intestinal tract in a way which prevents the lactase enzyme from being expressed.
Some studies indicate that environmental factors (more specifically, the consumption of lactose) may "play a more important role than genetic factors in the etio-pathogenesis of milk intolerance",[6] but some other publications suggest that lactase production does not seem to be induced by dairy/lactose consumption.[69]
Nutritional concerns
Primary lactose intolerance
Populations where primary lactose intolerance is the norm have demonstrated similar health levels to westerners (outside of malnutrition issues; see the History of genetic prevalence subsection above), or better health.
Secondary lactose intolerance
Dairy products are relatively good and accessible sources of calcium and potassium and many countries mandate that milk be fortified with vitamin A and vitamin D. Consequently, in dairy-consuming societies, dairy is often a main source of these nutrients and, for lacto-vegetarians, a main source of vitamin B12. Individuals who reduce or eliminate consumption of dairy must obtain these nutrients elsewhere. However, Asian populations for whom dairy is not part of their food culture do not present decreased health and sometimes present above average health, as in Japan.
Plant based milk substitutes are not naturally rich in calcium, potassium, or vitamins A or D (and, like most non-animal products, contain no vitamin B12). However, prominent brands are often voluntarily fortified with many of these nutrients.
An increasing number of calcium-fortified breakfast foods — such as orange juice, bread, and dry cereal — have been appearing on supermarket shelves. Many fruits and vegetables are rich in potassium and vitamin A; animal products like meat and eggs are rich in vitamin B12, and the human body itself produces some vitamin D from exposure to direct sunlight. Finally, a dietitian or physician may recommend a vitamin or mineral supplement to make up for any remaining nutritional shortfall.
Lactose-reduced dairy products have the same nutritional content as their full-lactose counterparts, but their taste and appearance may differ slightly.
Most infants with gastroenteritis due to rotavirus do not develop lactose intolerance,[70] so these infants do not benefit from being put on a lactose-free diet unless symptoms of lactose intolerance are severe and persistent.
Congenital lactase deficiency
Congenital lactase deficiency, or CLD, is an autosomal recessive disorder which prevents the expression of lactase.[71] Before the 20th century, infants with this disease rarely survived. As substitute and lactose-free infant formulas later became available, nursing infants affected with CLD could now have their normal nutritional needs met. Beyond infancy, individuals with CLD usually have the same nutritional concerns as those affected by secondary lactose intolerance.
See also
- Food allergy
- Gastroenterology
- Gluten intolerance
- Dairy allergy
- Soy milk
- Soy cheese
- Sucrose intolerance
References
- Durand, P. (1959). "Lactosurie et saccharosurie". In Ed. E. Rossi, E. Gautier, and J. W. Weber. Paediat. IV. Carbohydrate Metabolism in Children. Basel. pp. 496–502.
- Holzel A, Schwarz V, Sutcliffe KW (1959). "Defective lactose absorption causing malnutrition in infancy". Lancet 1 (7083): 1126–8. doi:10.1016/S0140-6736(59)90710-X. PMID 13665980.
- Carroccio A, Montalto G, Cavera G, Notarbatolo A (1998). "Lactose intolerance and self-reported milk intolerance: relationship with lactose maldigestion and nutrient intake. Lactase Deficiency Study Group". J Am Coll Nutr 17 (6): 631–6. PMID 9853544. http://www.jacn.org/cgi/content/full/17/6/631.
- McGee, Harold (2004). "Milk after infancy: dealing with lactose". On food and cooking: the science and lore of the kitchen. New York: Scribner. pp. 14–15. ISBN 0-684-80001-2.
- Rusynyk RA, Still CD (2001). "Lactose intolerance" (PDF). J Am Osteopath Assoc 101 (4 Suppl Pt 1): S10–2. PMID 11392211. http://www.jaoa.org/cgi/reprint/101/4_suppl_1/10S.
Notes
- ↑ "Improved lactose digestion and intolerance among African-American adolescent girls fed a dairy-rich diet.". Journal of the American Dietetic Association. 2000. http://www.accessmylibrary.com/coms2/summary_0286-27939567_ITM. Retrieved 2009-02-03. "Approximately 75% of the world's population loses the ability to completely digest a physiological dose of lactose after infancy".
- ↑ Bulhoes, A. C., et al. (2007-11). "Correlation between lactose absorption and the C/T-13910 and G/A-22018 mutations of the lactase-phlorizin hydrolase (LCT) gene in adult-type hypolactasia". Brazilian Journal of Medical and Biological Research. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-879X2007001100004&lng=en&nrm=iso&tlng=en. Retrieved 2008-07-19.
- ↑ Soy and Lactose Intolerance Wayback: Soy Nutrition
- ↑ Coles Harriet (2007-01-20). "The lactase gene in Africa: Do you take milk?". The Human Genome, Wellcome Trust. http://genome.wellcome.ac.uk/doc_WTX038968.html. Retrieved 2008-07-18.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Järvelä I (2002). "Identification of a variant associated with adult-type hypolactasia". Nat. Genet. 30 (2): 233–7. doi:10.1038/ng826. PMID 11788828.
- ↑ 6.0 6.1 6.2 Yoshida Y, Sasaki G, Goto S, Yanagiya S, Takashina K (1975). "Studies on the etiology of milk intolerance in Japanese adults". Gastroenterol. Jpn. 10 (1): 29–34. PMID 1234085.
- ↑ N.S. Enattah et al. (1, September 2007). "Evidence of Still-Ongoing Convergence Evolution of the Lactase Persistence T-13910 Alleles in Humans". American Journal of Human Genetics. http://www.ajhg.org/AJHG/fulltext/S0002-9297(07)61358-5.
- ↑ 8.0 8.1 Flatz G (1987). "Genetics of lactose digestion in humans". Adv. Hum. Genet. 16: 1–77. PMID 3105269.
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 Kretchmer N (1972). "Lactose and lactase". Sci. Am. 227 (4): 71–8. PMID 4672311.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 10.8 "Lactose Intolerance: The Molecular Explanation". UC Davis Nutritional Genomics. http://nutrigenomics.ucdavis.edu/nutrigenomics/index.cfm?objectid=968814F6-65B3-C1E7-0C7007B71CC9959A.
- ↑ Anne Charlotte Jäger (1, February 2006). "Laktose-intolerans: Gentest for laktose-intolerans - hurtig og billig diagnostik". DSKB-NYT. http://www.dskb.dk/index.jsp?id=7353&gr=7446.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 de Vrese M, Stegelmann A, Richter B, Fenselau S, Laue C, Schrezenmeir J (2001). "Probiotics--compensation for lactase insufficiency". Am. J. Clin. Nutr. 73 (2 Suppl): 421S–429S. PMID 11157352. http://www.ajcn.org/cgi/content/full/73/2/421S.
- ↑ G.D. Smith et al., Lactase persistence-related genetic variant: population substructure and health outcomes. European Journal of Human Genetics, 2008.
- ↑ Valenkevich LN, Iakhontova OI (2005). "[Prevalence of the lactase deficiency among the population of the northwestern region of Russia]" (in Russian). Eksp Klin Gastroenterol (1): 97–100, 108. PMID 15991859.
- ↑ http://yannklimentidis.blogspot.com/2007/03/monday-maps-lactase-persistence-in.html
- ↑ 16.0 16.1 16.2 Cavalli-Sforza LT, Strata A, Barone A, Cucurachi L (1987). "Primary adult lactose malabsorption in Italy: regional differences in prevalence and relationship to lactose intolerance and milk consumption" (PDF). Am. J. Clin. Nutr. 45 (4): 748–54. PMID 3565303. http://www.ajcn.org/cgi/reprint/45/4/748.pdf.
- ↑ (Portuguese) Intolerância à lactose Maria do Céu Salgado - Outubro de 2007
- ↑ Kozlov A, Lisitsyn D (1997). "Hypolactasia in Saami subpopulations of Russia and Finland". Anthropol Anz 55 (3-4): 281–7. PMID 9468755.
- ↑ Jackson RT, Latham MC (1979). "Lactose malabsorption among Masai children of East Africa". Am. J. Clin. Nutr. 32 (4): 779–82. PMID 581925.
- ↑ 20.0 20.1 20.2 Ernest L. Abel (2001-08). Jewish Genetic Diseases: a Layman's Guide. McFarland & Company, Inc., Publishers. ISBN 9780786409419. http://books.google.com/?id=yUNKaRAVZvMC&pg=PA156&lpg=PA156&dq=lactose+intolerance+among+jews&q=lactose%20intolerance%20among%20jews.
- ↑ Burgio GR, Flatz G, Barbera C, et al. (1984). "Prevalence of primary adult lactose malabsorption and awareness of milk intolerance in Italy" (PDF). Am. J. Clin. Nutr. 39 (1): 100–4. PMID 6691285. http://www.ajcn.org/cgi/reprint/39/1/100.pdf.
- ↑ Vesa TH, Marteau P, Korpela R (2000). "Lactose intolerance". J Am Coll Nutr 19 (2 Suppl): 165S–175S. PMID 10759141. http://www.jacn.org/cgi/content/full/19/suppl_2/165S.
- ↑ 23.0 23.1 23.2 Wang YG, Yan YS, Xu JJ, et al. (1984). "Prevalence of primary adult lactose malabsorption in three populations of northern China". Hum. Genet. 67 (1): 103–6. doi:10.1007/BF00270566. PMID 6235167.
- ↑ Nasrallah SM (1979). "Lactose intolerance in the Lebanese population and in "Mediterranean lymphoma"" (PDF). Am. J. Clin. Nutr. 32 (10): 1994–6. PMID 484518. http://www.ajcn.org/cgi/reprint/32/10/1994.pdf.
- ↑ 25.0 25.1 Sahi T (1994). "Genetics and epidemiology of adult-type hypolactasia". Scand. J. Gastroenterol. Suppl. 202: 7–20. doi:10.3109/00365529409091740. PMID 8042019.
- ↑ Woteki CE, Weser E, Young EA (1976). "Lactose malabsorption in Mexican-American children" (PDF). Am. J. Clin. Nutr. 29 (1): 19–24. PMID 946157. http://www.ajcn.org/cgi/reprint/29/1/19.pdf.
- ↑ Swallow DM (2003). "Genetics of lactase persistence and lactose intolerance". Annu. Rev. Genet. 37: 197–219. doi:10.1146/annurev.genet.37.110801.143820. PMID 14616060.
- ↑ Matthews SB, Waud JP, Roberts AG, Campbell AK (2005). "Systemic lactose intolerance: a new perspective on an old problem". Postgrad Med J 81 (953): 167–73. doi:10.1136/pgmj.2004.025551. PMID 15749792.
- ↑ R. Bowen (December 28, 2006). "Lactose Intolerance (Lactase Non-Persistence)". Pathophysiology of the Digestive System. Colorado State University. http://www.vivo.colostate.edu/hbooks/pathphys/digestion/smallgut/lactose_intol.html.
- ↑ National Digestive Diseases Information Clearinghouse (March 2006). "Lactose Intolerance -- How is lactose intolerance diagnosed?". National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. http://digestive.niddk.nih.gov/ddiseases/pubs/lactoseintolerance/#diagnosed.
- ↑ 31.0 31.1 31.2 Hargrove, James L.; Berdanier, Carolyn D. (1993). Nutrition and gene expression. Boca Raton: CRC Press. ISBN 0-8493-6961-4.
- ↑ Wilson J (December 2005). "Milk Intolerance: Lactose Intolerance and Cow's Milk Protein Allergy". Newborn and Infant Nursing Reviews 5 (4): 203–7. doi:10.1053/j.nainr.2005.08.004.
- ↑ Joseph Jordania (2006). Who Asked the First Question? The Origins of Human Choral Singing, Intelligence, Language and Speech. Tbilisi: Logos. ISBN 99940-31-81-3.
- ↑ Swaminathan, N. 2007. Not Milk? Neolithic Europeans Couldn't Stomach the Stuff. Scientific American.
- ↑ Malmstrom, H., Linderholm, A., Liden, K., Stora, J., Molnar, P., Holmlund, G., Jakkobson, M., Gotherstrom, A. 2010. High frequency of lactose intolerance in a prehistoric hunter-gatherer population in northern Europe. BMC Evolutionary Biology 10: 89.
- ↑ Coelho, M., Luiselli, D., Bertorelle, G., Lopes, A. I., Seixas, S., Destro-Bisol, G. and Rocha, J. 2002. Microsatellite variation and evolution of human lactase persistence. Human Genetics 117(4): 329–339.
- ↑ Bersaglieri T., Sabeti P. C., Patterson N., Vanderploeg T., Schaffner S. F., Drake J. A., Rhodes M., Reich D. E. and Hirschhorn J. N. 2004. Genetic signatures of strong recent positive selection at the lactase gene. American Journal of Human Genetics 74(6): 1111–20.
- ↑ 38.0 38.1 Wade, N. Study Detects Recent Instance of Human Evolution. The New York Times. December 10 2006.
- ↑ 39.0 39.1 Swaminathan, N. 2006. African Adaptation to Digesting Milk Is "Strongest Signal of Selection Ever". Scientific American.
- ↑ Aoki, K. 2001. Theoretical and Empirical Aspects of Gene–Culture Coevolution. Theoretical Population Biology 59(4): 253–261.
- ↑ 41.0 41.1 41.2 Swallow, D. M. 2003. Genetics of Lactase Persistence and Lactose Intolerance. Annual Review of Genetics 37: 197–219.
- ↑ Milk Drinking Started Around 7,500 Years Ago In Central Europe, Science Daily, Sep. 1, 2009
- ↑ Myles, S., Bouzekri, N., Haverfield, E., Cherkaoui, M., Dugoujon, J. M. and Ward, R. 2005. Genetic evidence in support of a shared Eurasian-North African dairying origin. Biomedical and Life Sciences 117(1): 34–42.
- ↑ Tishkoff, S. A., Reed, F. A., Ranciaro, A., Voight, B. F., Babbitt, C. C., Silverman, J. S., Powell, K., Mortensen, H. M., Hirbo, J. B., Osman, M., Ibrahim, M., Omar, S. A., Lema, G., Nyambo, T. B., Ghori, J., Bumpstead, S., Pritchard, J. K., Wray, G. A. and Deloukas, P. 2006. Convergent adaptation of human lactase persistence in Africa and Europe. Nature Genetics 39: 31–40.
- ↑ Enattah, N. S., Jensen, T. G. K., Nielsen, M., Lewinski, R., Kuokkanen, M., Rasinpera, H., El-Shanti, H., Kee Seo, J., Alifrangis, M., Khalil, I. F., Natah, A., Ali, A., Natah, S., Comas, D., Mehdi, S. Q., Groop, L., Vestergaard, E. M., Imtiaz, F., Rashed, M. S., Meyer, B., Troelsen, J., and Peltonen, L. 2008. Independent Introduction of Two Lactase-Persistence Alleles into Human Populations Reflects Different History of Adaptation to Milk Culture. American Journal of Human Genetics 82(1): 57–72.
- ↑ Itan, Y., Jones, B. L., Ingram, C. J. E., Swallow, D. M. and Thomas, M. G. 2010. A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evolutionary Biology 10: 36.
- ↑ Itan, Y., Powell, A., Beaumont, M. A., Burger, J., Thomas, M. G. 2009. The Origins of Lactase Persistence in Europe. PLoS Computational Biology 5(8): e1000491.
- ↑ "Lactose tolerance/intolerance". Gene Expression. January 19, 2004. http://www.gnxp.com/MT2/archives/001681.html. Retrieved 2008-01-31.
- ↑ Lactose Intolerance at eMedicine Roy, Barakat, Nwakakwa, Shojamanesh, Khurana, July 5, 2006 - About 44% of lactose intolerant women regain the ability to digest lactose during pregnancy. This might be caused by slow intestinal transit and intestinal flora changes during pregnancy.
- ↑ 50.0 50.1 National Digestive Diseases Information Clearinghouse (March 2006). "Lactose Intolerance". National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. http://digestive.niddk.nih.gov/ddiseases/pubs/lactoseintolerance/.
- ↑ Composition of Human, Cow, and Goat Milks - Goat Milk - GOATWORLD.COM
- ↑ Peeva (2001). "Composition of buffalo milk. Sources of specific effects on the separate components". Bulg. J. Agric. Sci. 7: 329–35. http://bjas.hit.bg/07/693A.htm.
- ↑ C:\JAG2\Jiang.vp
- ↑ http://www.ilovecheese.com/lactose_intolerant_faqs.asp
- ↑ Dairy Science and Technology, Dept. of Food Science. "Ice Cream Formulations". University of Guelph. http://www.foodsci.uoguelph.ca/dairyedu/icform.html.
- ↑ Reger, Combs, Coulter and Koch (February 1, 1951). "A Comparison of Dry Sweet Cream Buttermilk and Non-Fat Dry Milk Solids in Breadmaking". Journal of Dairy Science 34 (2): 136–44. doi:10.3168/jds.S0022-0302(51)91682-7. http://jds.fass.org/cgi/content/abstract/34/2/136.
- ↑ Goat Milk Composition
- ↑ 58.0 58.1 "General guidelines for milk allergy". Oregon Health & Science University. http://www.ohsu.edu/xd/health/health-information/topic-by-id.cfm?ContentTypeId=90&ContentId=P01696.
- ↑ "Margarine Regulations". http://www.gov.ns.ca/JUST/REGULATIONS/regs/marge.htm.
- ↑ "Enriched White Bread in Canada". The Canadian Celiac Association. http://www.celiac.ca/Articles/PAB%20Enriching%20GF%20Foods.html.
- ↑ Riggs, Lloyd K; Beaty, Annabel; Johnson, Arnold H (December). "Influence of Nonfat Dry Milk Solids on the Nutritive Value of Bread". Journal of Dairy Science 29 (12): 821–9. doi:10.3168/jds.S0022-0302(46)92546-5. http://jds.fass.org/cgi/content/abstract/29/12/821.
- ↑ "Bartek, food additive company" (PDF). http://www.bartek.ca/pdfs/Applications/SavouryProducts/SavourySnackFoods/Savoury%20Snack%20Foods%20Alphabetical%20List%20of%20Product%20Names.pdf.
- ↑ Sahi T. (1974 PMID: 4852638). "Lactose malabsorption in Finnish-speaking and Swedish-speaking populations in Finland". Scand J. Gastroenterol. 9 (3): 303–8. PMID 4852638. http://www.ncbi.nlm.nih.gov/pubmed/4852638.
- ↑ Montalto M, Curigliano V, Santoro L, et al. (2006). "Management and treatment of lactose malabsorption". World J. Gastroenterol. 12 (2): 187–91. PMID 16482616. http://www.wjgnet.com/1007-9327/12/187.asp.
- ↑ He M, Yang Y, Bian L, Cui H (1999). "[Effect of exogenous lactase on the absorption of lactose and its intolerance symptoms]" (in Chinese). Wei Sheng Yan Jiu 28 (5): 309–11. PMID 12712706.
- ↑ O'Connell S, Walsh G (2006). "Physicochemical characteristics of commercial lactases relevant to their application in the alleviation of lactose intolerance". Appl. Biochem. Biotechnol. 134 (2): 179–91. doi:10.1385/ABAB:134:2:179. PMID 16943638.
- ↑ Heyman MB; Committee On, Nutrition (2006). "Lactose intolerance in infants, children, and adolescents". Pediatrics 118 (3): 1279–86. doi:10.1542/peds.2006-1721. PMID 16951027. http://pediatrics.aappublications.org/cgi/content/full/118/3/1279.
- ↑ Lactose intolerant? Drink more milk Steve Tally
- ↑ Prevalence, Age & Genetics of Lactose Intolerance - foodreactions.org
- ↑ Turck D (2007). "[Prevention and treatment of acute diarrhea in infants]" (in French). Arch Pediatr 14 (11): 1375–8. doi:10.1016/j.arcped.2007.06.009. PMID 17629685.
- ↑ Lactose Intolerance at eMedicine Guandalini S, Frye R, Rivera-Hernández D, Miller L, Borowitz S
External links
- United States National Institutes of Health page regarding lactose intolerance
- Scientific American: African Adaptation to Digesting Milk Is "Strongest Signal of Selection Ever" (East African cattle herding communities rapidly and independently evolved ability to digest lactose)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||